The Essential Guide to Lean Management: Transforming Organizations Through Continuous Improvement
What if you could reclaim one working day each week—all while delighting customers and empowering your team? This isn’t a fantasy. It’s the reality for thousands of organizations that have embraced Lean Management principles. In an era where efficiency, quality, and customer satisfaction determine competitive advantage, Lean has emerged as more than just a methodology—it’s […]
Implementing Change Control Management in ISO 13485
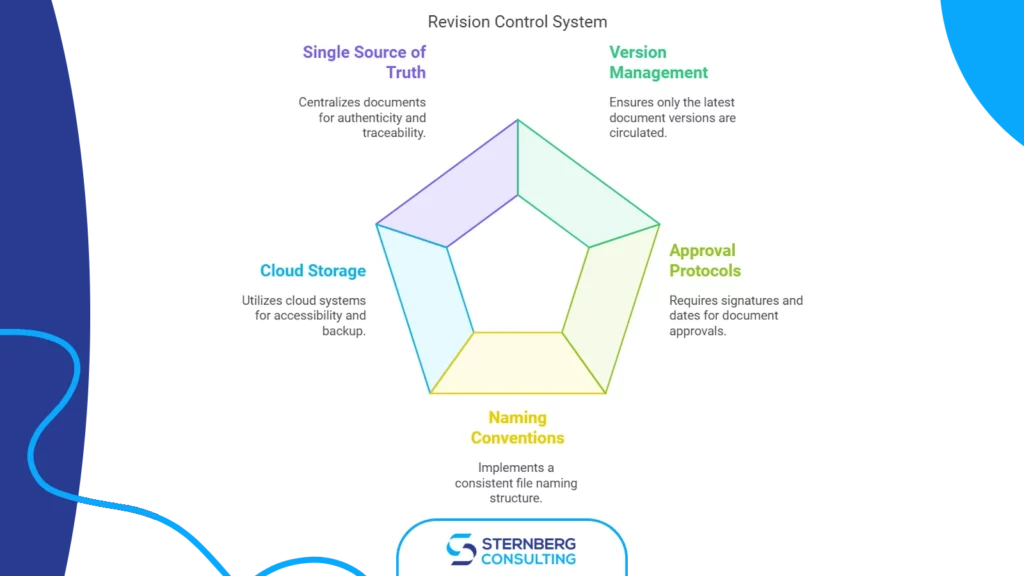
Change is inevitable, but in the medical device industry, it’s also an opportunity— to innovate, refine, and build trust. Implementing change control management in ISO 13485 systems transforms this constant flux into a well-orchestrated symphony of quality, safety, and compliance. Whether it’s updating a design, optimizing a process, or collaborating with a new supplier, robust […]
Managing Change Control in ISO 13485

Change control is a vital aspect of quality management for medical device companies. ISO 13485, the international standard for medical device quality management systems, places emphasis on change management. The process of change control in ISO 13485 ensures that modifications to products, processes, or documentation are implemented in a controlled manner. This helps maintain product […]
Feedback Instead of Complaints? The Key Differences

Have you ever wondered if it might be possible to simply move a complaint into a less burdensome category like feedback? This thought can easily arise when quality goals are narrowly missed and the pressure to deliver good metrics grows. That’s exactly what I came across recently: the question of whether complaints could be declared […]
