ISO 9001:2026 — Prepare for the New Requirements
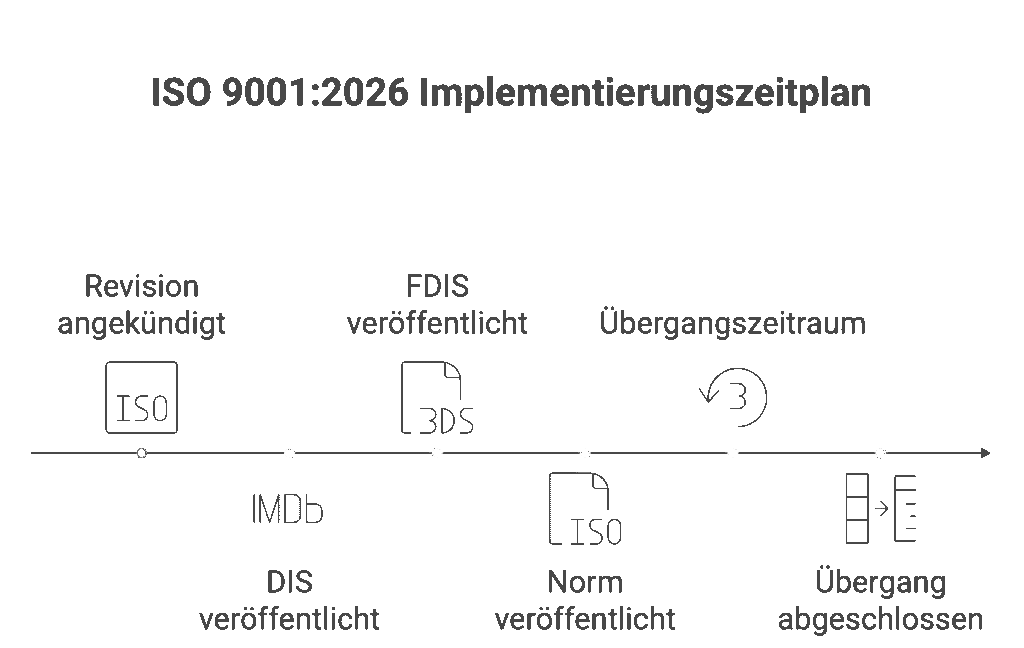
Introduction: Why is the 2026 Revision Coming? The quality management standard ISO 9001 will be revised in 2026, bringing it up to date for the first time in more than ten years. The current ISO 9001:2015 was published in 2015 – since then, technologies, business models, and expectations have evolved significantly. An international survey in […]
How to Make Supplier Evaluation Practical and Risk-Based

Supplier evaluation is a topic where many companies unintentionally create unnecessary complexity. Long forms are sent to suppliers asking about their annual revenue, number of employees, ownership structures, and countless other details that often have no direct impact on the actual business relationship. Internal teams then spend hours collecting and filing these documents, while suppliers […]
The Essential Guide to Lean Management: Transforming Organizations Through Continuous Improvement

What if you could reclaim one working day each week—all while delighting customers and empowering your team? This isn’t a fantasy. It’s the reality for thousands of organizations that have embraced Lean Management principles. In an era where efficiency, quality, and customer satisfaction determine competitive advantage, Lean has emerged as more than just a methodology—it’s […]
Implementing Change Control Management in ISO 13485
Change is inevitable, but in the medical device industry, it’s also an opportunity— to innovate, refine, and build trust. Implementing change control management in ISO 13485 systems transforms this constant flux into a well-orchestrated symphony of quality, safety, and compliance. Whether it’s updating a design, optimizing a process, or collaborating with a new supplier, robust […]
Managing Change Control in ISO 13485

Change control is a vital aspect of quality management for medical device companies. ISO 13485, the international standard for medical device quality management systems, places emphasis on change management. The process of change control in ISO 13485 ensures that modifications to products, processes, or documentation are implemented in a controlled manner. This helps maintain product […]
Feedback Instead of Complaints? The Key Differences

Have you ever wondered if it might be possible to simply move a complaint into a less burdensome category like feedback? This thought can easily arise when quality goals are narrowly missed and the pressure to deliver good metrics grows. That’s exactly what I came across recently: the question of whether complaints could be declared […]
ISO 13485 Clause 4.1: General Quality Management System Requirements

Review ISO 13485 – Clause 4.1 to get familiar with General QMS Requirements for companies in the medical device industry. We’ll cover documentation needs, processes, risk management, and much more.
How to Get ISO 13485 Certification: A Step-by-Step Guide

In medical device manufacturing, getting ISO 13485 certification ensures quality and regulatory compliance. This international standard sets the bar for quality management systems in the medical device industry. Companies seeking to enhance their processes, meet regulatory requirements, and gain a competitive edge often ask how to get ISO 13485 certification. It requires careful preparation and […]
ISO 13485 and Regulatory Requirements: Complete Compliance

ISO 13485 is an international standard for quality management systems in the medical device industry. This is essential for medical device companies aiming to ensure their products consistently meet regulatory and customer requirements. So, let’s explore the connection between ISO 13485 and regulatory requirements. Walking down this path can help ensure regulatory compliance of your […]
Check Sheet – Data Collection Tool

In the realm of quality control and data collection, the check sheet stands as a fundamental tool for professionals seeking precision and efficiency. This simple yet powerful instrument enables organizations to gather, organize, and analyze data systematically, providing a solid foundation for informed decision-making. Check sheets have proven invaluable across various industries, from manufacturing to […]

